Calculating Hydronium ion concentration from pH (and identifying molecules based on pH) : r/chemhelp

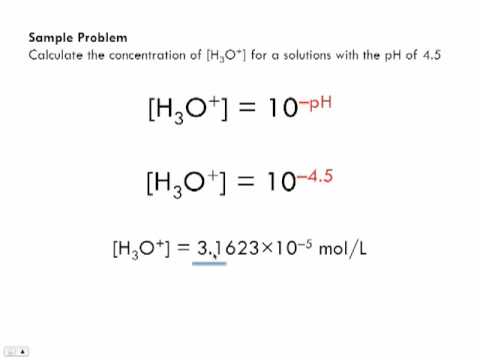
Calculate the hydrogen ion concentration in the following biological fluids whose pH are given below:(a) Human muscle - fluid, 6.83 (b) Human stomach fluid, 1.2 (c) Human blood, 7.38 (d) Human saliva, 6.4.
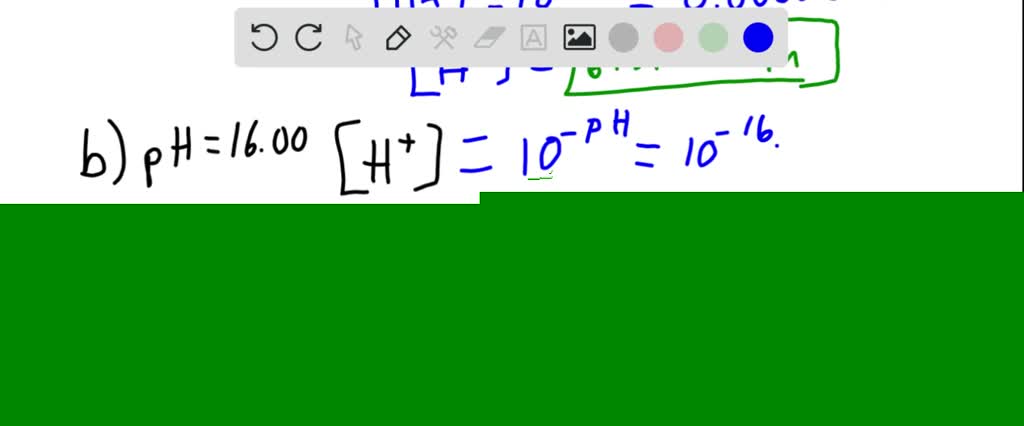
SOLVED:Calculate the hydrogen ion concentration in mol/L for each of the following solutions: (a) a solution whose pH is 5.20, (b) a solution whose pH is 16.00,(c) a solution whose hydroxide concentration
![SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice, SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice,](https://cdn.numerade.com/previews/c92ef7f1-c83f-4027-9f05-6b11ebe1b21e_large.jpg)
SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice,
![Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. Calculate Ka/Kb, given the pH or pOH and the concentration. - ppt download Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. Calculate Ka/Kb, given the pH or pOH and the concentration. - ppt download](https://images.slideplayer.com/34/8310807/slides/slide_10.jpg)
Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. Calculate Ka/Kb, given the pH or pOH and the concentration. - ppt download
![Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download](https://images.slideplayer.com/17/5302527/slides/slide_3.jpg)
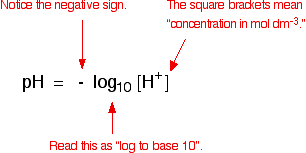
Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download







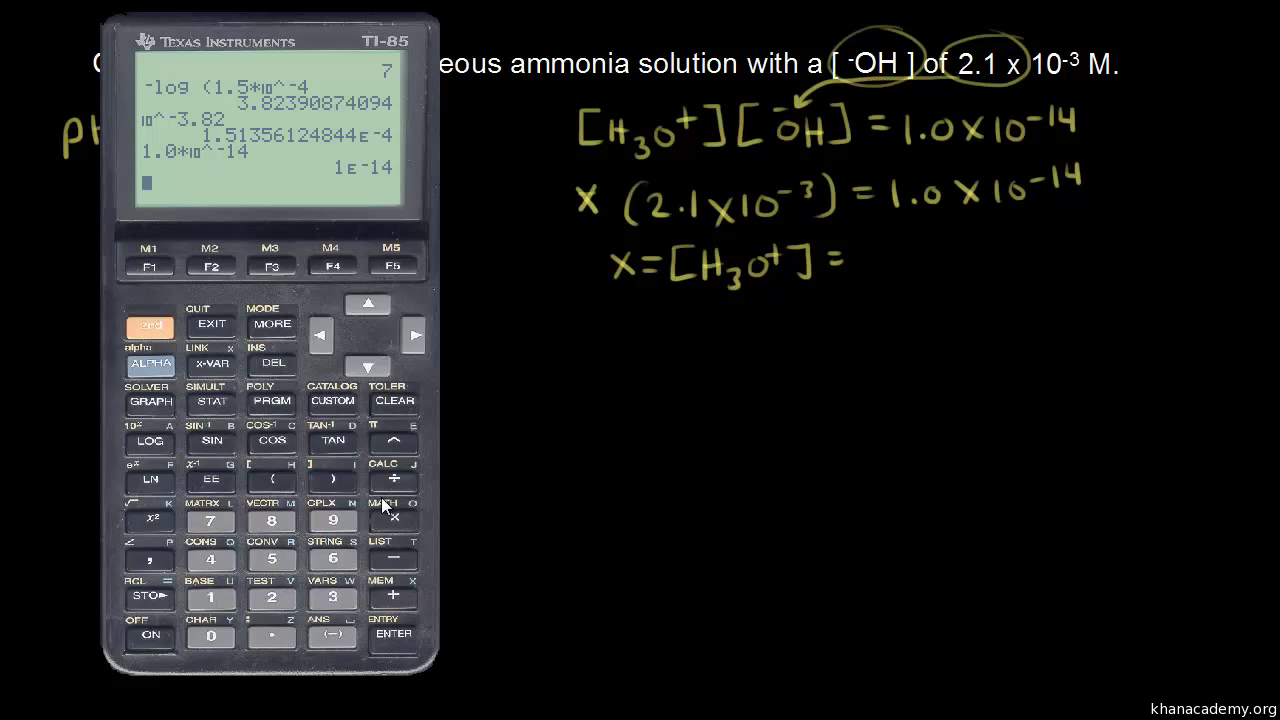
![Calculating [H+] from pH, Acids & Bases Tutorial - YouTube Calculating [H+] from pH, Acids & Bases Tutorial - YouTube](https://i.ytimg.com/vi/bP-evPgNNUg/maxresdefault.jpg)